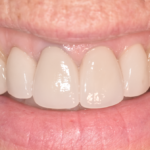
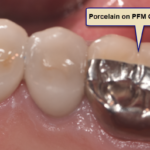
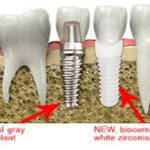
Amalgam Fillings
This week I will be posting excerpts from articles and commenting on the July 28th ruling from the FDA on mercury and dental amalgam safety. The new classification and comments on their website was somewhat of a disappointment to say the least. This was a turnaround after the settlement that was reached with Charlie Brown, the attorney from Consumers for Dental Choice, in the summer of 2008.
Here is what the FDA has on their website as of July 28th, 2009:
There is limited clinical information about the potential effects of dental amalgam fillings on pregnant women and their developing fetuses, and on children under the age of 6, including breastfed infants. However, the estimated amount of mercury in breast milk attributable to dental amalgam is low and falls well below general levels for oral intake that the Environmental Protection Agency (EPA) considers safe. FDA concludes that the existing data support a finding that infants are not at risk for adverse health effects from the breast milk of women exposed to mercury vapor from dental amalgam. The estimated daily dose of mercury vapor in children under age 6 with dental amalgams is also expected to be at or below levels that the EPA and the Centers for Disease Control and Prevention (CDC) consider safe. Pregnant or nursing mothers and parents with young children should talk with their dentists if they have concerns about dental amalgam.
This is a significant change and reversal from their position last summer. It was last June that the FDA settled the lawsuit and agreed to withdraw claims of amalgam safety from their web site and posted the following warning, “Dental amalgams contain mercury, which may have neurotoxic effects on the nervous system of developing children and fetuses.”
Dr. Carey O’Rielly is a holistic dentist in San Diego, California who truly understands and practices the tenets of holistic health, both as a practitioner and in his personal life. His remarkable knowledge of the field came about through years of study and experience, initiated by his own work-related health challenges.